A new Luminol based formula
BLUESTAR® FORENSIC is a blood visualizing agent based on luminol, a molecule well-known to forensic experts.
Unlike older formulations, BLUESTAR® eliminates many of the drawbacks associated with traditional luminol-based reagents.
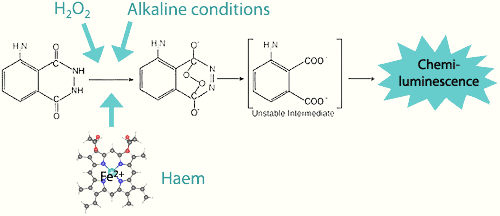
How does Luminol React ?
Luminol (3-Aminophthalhydrazide) was first synthesized in 1853. Its chemiluminescent property was discovered in 1928 by Albrecht: in a basic solution and with an oxidizing agent, luminol emits a blue glow when in contact with blood.
The light-emitting reaction is catalyzed by the iron in hemoglobin, through the activity of the haem group — also found in peroxidase enzymes.
Thus, when luminol + oxidizer + alkaline agent come into contact with blood, a bright blue light is emitted.

From Luminol to Bluestar® Forensic: a brief history
- 1937 – Specht pioneers the use of luminol on natural surfaces (lawn, bricks, stones).
- 1939 – Proesher & Moody validate the reaction on human and animal blood.
- 1951 – Grodsky creates a first-generation kit using luminol, sodium carbonate, and sodium perborate. The reaction was weak, short-lived, and the formula toxic and unstable.
- 1966 – Weber proposes an improved formula with sodium hydroxide and hydrogen peroxide. This mix had to be kept cold and only worked in total darkness.
The BLUESTAR® Breakthrough
In 2000, Jean-Marc Lefebvre-Despeaux, president of BLUESTAR®, tasked Prof. Loïc Blum, biochemistry professor at Claude Bernard University (Lyon) and director of the CNRS-UCBL EMB2 lab, with the development of a new, safer and more stable luminol-based formula.
The result was BLUESTAR® FORENSIC:
A revolutionary reagent used today in over 95 countries and recognized as the most effective tool for detecting blood traces — even ancient and minute ones — without compromising DNA integrity.
